

Our Rough Guide to Crystallography
Why Study Crystal Structures?
The physical properties of solids depend entirely upon the arrangement of the atoms that make up the solid and the distances between them.
Thus, structures such as diamond, with strong bonds between the atoms tend to be very hard materials. Structures with weaker bonding, such as NaCl (salt), are softer and easier to break.
The arrangement of atoms can also give rise to useful electrical and physical properties. For example, the specific arrangement of the silicon and oxygen atoms in quartz give rise to the property of peizoelectricity. This means that when the quartz crystal is physically deformed an electric charge is developed across the crystal. This is the basis of the quartz oscillator found in wrist-watches.
Organic and metal-organic molecules form crystals that contain isolated molecules, usually held together by weak inter-molecular forces such as hydrogen bonds or van-der-Waals interactions.
Most biological molecules such as proteins, enzymes and even DNA can be crystallised. Determining the arrangement of atoms within these crystals tell biochemists how these molecules, and thus our bodies,function. Crystallography is also an important step in designing drugs to combat specific diseases.
Thus, structures such as diamond, with strong bonds between the atoms tend to be very hard materials. Structures with weaker bonding, such as NaCl (salt), are softer and easier to break.
The arrangement of atoms can also give rise to useful electrical and physical properties. For example, the specific arrangement of the silicon and oxygen atoms in quartz give rise to the property of peizoelectricity. This means that when the quartz crystal is physically deformed an electric charge is developed across the crystal. This is the basis of the quartz oscillator found in wrist-watches.
Organic and metal-organic molecules form crystals that contain isolated molecules, usually held together by weak inter-molecular forces such as hydrogen bonds or van-der-Waals interactions.
Most biological molecules such as proteins, enzymes and even DNA can be crystallised. Determining the arrangement of atoms within these crystals tell biochemists how these molecules, and thus our bodies,function. Crystallography is also an important step in designing drugs to combat specific diseases.
How we determine Crystal Structures?
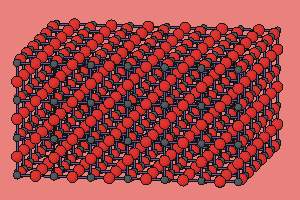 |
Most crystal structures consist of a regular, periodic array of atoms.
This is a simple structure consisting of six oxygen atoms surrounding each metal atom. The structure is periodic, which means that it is identical at all points within the crystal. |
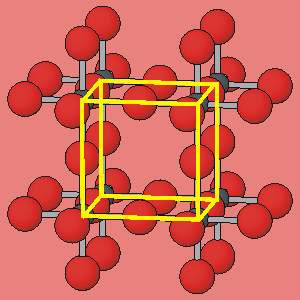 |
Because of the periodicity, the structure can be described in terms of unit-cells, all of which are identical.
The unit cell of this structure is outlined in this detail of the structure. The whole crystal structure is built by stacking these identical unit-cells together. The periodicity of crystal structures means that they can act as a diffraction grating for X-rays - hence "X-ray diffraction". |
The arrangement of the atoms in a crystal structure is a combination of the unit-cell size and shape and the arrangement of atoms inside the unit-cell.
Arrangement of atoms
The arrangement of atoms within the unit-cell can be described in terms of their fractional coordinates or positions relative to the corners of the unit-cell.
The intensities of X-ray beams diffracted by a crystal structure are related to the fractional coordinates of the atoms.
We use the Xcalibur diffractometers (Xcalibur1 and Xcalibur2) to measure the intensities of X-ray beams diffracted from a crystal to determine the arrangement of atoms within the unit-cell.
See the structures page for more details.
The intensities of X-ray beams diffracted by a crystal structure are related to the fractional coordinates of the atoms.
We use the Xcalibur diffractometers (Xcalibur1 and Xcalibur2) to measure the intensities of X-ray beams diffracted from a crystal to determine the arrangement of atoms within the unit-cell.
See the structures page for more details.
Unit cell size and shape
The shape of the unit cells is described by the lattice symmetry. Thus, this unit cell is cubic because the edge lengths are equal and the angles between the edges are all 90 degrees.
The size of the unit-cell is described in terms of its unit-cell parameters. These are the edge lengths and the angles of the unit-cell.
The positions of the X-ray beams diffracted by a crystal structure are related to the size and shape of the unit-cell.
We use the Huber diffractometer to measure the positions of X-ray beams diffracted from a crystal to determine the size and shape of the unit-cell.
See the lattice parameters page for more details.
The size of the unit-cell is described in terms of its unit-cell parameters. These are the edge lengths and the angles of the unit-cell.
The positions of the X-ray beams diffracted by a crystal structure are related to the size and shape of the unit-cell.
We use the Huber diffractometer to measure the positions of X-ray beams diffracted from a crystal to determine the size and shape of the unit-cell.
See the lattice parameters page for more details.