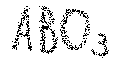
Classification of Perovskites by Chemical Criteria
A possible classification - and a quite logical one, as we are chemists - regards the different anions, that are found in this structure type.
There are several requirements for the anions (cf. the section dealing with concepts for the prediction of structural distorsion, too):
- Relatively low ionic radii in the range of those of the biggest cations known in the periodic system.
- Relatively low polarizability in order to prevent the formation of covalent bonds.
Thus, only few anions remain that fulfill the demands, e.g. O2-, S2-, F- and Cl-. A mixture of different anoins is possible (e.g. Oxyfluorides). The largest groups of perovskite type mixed metal oxides are namely the Fluoroperovskites and the Oxoperovskites.
Fluoroperovskites
The choice of cations that form Fluoroperovskites is limited to a I / II combination.
Monovalent cations are often found among Na, K, Rb, Cs, Ag, Tl. Typical divalent cations are Mg, Mn, Fe, Co, Ni, Cu, Zn. Due to the ionic radii, the monovalent cations usually participate in the AF3-layers whereas the smaller divalent cations occupy the octahedrally coordinated positions.
Perovskites with inverted site occupancy (an effect known from the spinel structure) are found when the divalent cation is larger of radius than the monovalent as in the case of BaLiF3.
Oxoperovskites
The mixed metal oxides can be further grouped by the charge of the cations. Given the stoichiometry ABO3, 6 negative charges of oxygen are to be compensated. This is realized in the following manner:
- I / V
- e.g. KTaO3, NaNbO3 etc.
- II / IV
- e.g. CaTiO3, PbZrO3 etc.
- III / III
- e.g. GdFeO3, LaRhO3 etc.
The possibility of relatively free substitution of certain cations in the structure lead to the mentioned variety of perovskite-type metal oxides. Substitutional routes include
- replacement of a cation by another cation of same valency.
- replacement of a cation by a combination of cations of different valency such that the net charge remains identical.
(e.g. 1 x III <--- 0.5 x IV + 0.5 x II)
- replacement of a cation by a cation of different valency such that the charge difference is compensated by a reduction or oxidation of the second metal ion present in the compounds.
(e.g. La1-xSrxMnO3)
The oxo-perovskites of the transition metals are furthermore tolerant to the formation of defects of all kinds, i.e.
- A1-xBO3 (= AB1+xO3)
- AB1-xO3 (= A1+xBO3)
- ABO3±x
last changed: Jul 16, 1998. © 1997‚1998 Carsten Schinzer.