Physical Chemistry of Protein Crystallization
EMBL Practical Course on Protein Expression, Purification and Crystallization
August 14th-20th, 2000 EMBL Outstation Hamburg, Germany
Bernhard Rupp
University of California, LLNL-BBRP, Livermore, CA 94551
Institut für Theoretische Chemie und Strukturbiologie der Universtät Wien, A 1090 Wien
© 2000 Bernhard Rupp
Thermodynamic stability in multi-component systems
Equilibrium and stability in G/x space
Pathways in different crystallization methods
Introduction
Protein crystallization can be viewed as a special case of phase separation in a thermodynamically non-ideal mixture controlled by kinetic parameters: The protein has to separate from aqueous solution and should form a distinct and hopefully, well ordered crystalline solid phase. We will therefore discuss the thermodynamics of non-ideal phase equilibria. Non-ideality leads to thermodynamic excess properties, which manifest themselves in fugacity, activity, and mixing enthalpies. Virial expansions relate non-ideality directly to coefficients interpretable as a measure for the interactions between protein and solvent. We will derive bimodal (solubility) and spinodal (decomposition) curves and construct the corresponding phase diagrams. Properties derived from thermodynamic parameters, including virial coefficients, are invariant for a given system and determine only the range of possible crystallization, without any predictive quality to its actual occurrence. The realization of a thermodynamically possible scenario is governed by kinetic parameters such as nucleation and crystal growth mechanisms, which are at the current state of the art unpredictable, but can be investigated and analyzed a posteriori to derive models and empirical parameters for crystallization processes.
Thermodynamic stability
Given that a protein solution represents a multi-component thermodynamic system, we need to investigate which criteria determine stability and what separates stable from non- or meta-stable regions in a given system. First, we will consider the most simple case, an ideal versus a non-ideal gas, to introduce basic stability criteria and virial expansions. Subsequently we will discuss the general concept of thermodynamic stability using the second Legendre transform G(T,P,n) of the generalized Gibbs fundamental equation to derive stability under variation of composition. In the final step, we shall use the partial molar Gibbs energy (chemical potential) to construct the phase diagrams as used to describe crystallization pathways in protein-solvent systems. Fortunately, although comprehensive, these basics are much less difficult to understand than it initially might appear.
Ideal systems
The simplest case of a thermodynamic system is a gas of ideal (dimensionless and non-interacting) particles in a cylinder with a reversible piston at a fixed temperature T. Varying pressure P exerted on the piston will reversibly compress or expand the gas to a certain volume V.
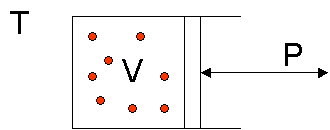
From the ideal gas law![]() follows that
follows that
![]() (1)
(1)
With T and n (the number of moles of gas) constant (R is already the gas constant, with a fixed numeric value of 8.3145 JK-1M-1) we see that the variation of volume vs. pressure is a simple hyperbolic function