The following figure shows what a certain resolution, given in ┼ngstr÷m (┼) means for the user of structural models derived from X-ray data. One always has to remember that the cute model you see was built into an experimental electron density. The model may look as good at 3 ┼ as it does at 1.5 ┼, but is it a correct and unique description of reality ?
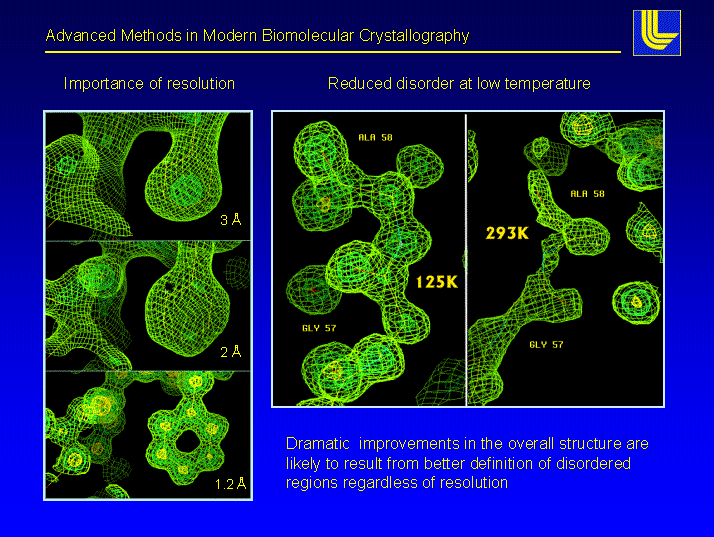
Above: pictures of the electron density at different data set resolution of the same region of a molecule. There is no question that a model of phenylalanine (the 6-ring structure) can be correctly placed into the 1.1 ┼ data. This still can be done with confidence in the 2 ┼ case, but at 3 ┼ we already observe a deviation of the centroid of the ring from the correct model.. The left panels shows the same nominal resolution structure at room temperature (293K) and nitrogen cooled (125K). The difference is a striking example for improvements that can be achieved using cryo-techniques.
Most protein crystals diffract between 1.8 and 3 ┼, a few to very high resolution (the term high resolution is used loosely in macromolecular crystallography, we apply it to data of 1.2 ┼ or better resolution). The most efficient way to increase resolution (short of trying to grow better crystals) is to cryo-cool the crystals to near liquid nitrogen temperature.
![]() Back to X-ray Tutorial Introduction
Back to X-ray Tutorial Introduction
LLNL Disclaimer
This World Wide Web site conceived and maintained by
Bernhard Rupp (br@llnl.gov)
Last revised April 04, 2000 10:12
UCRL-MI-125269