The thermal expansion has been made a function of temperature such that, following Pawley et al. (1996, Am Min), a single parameter a° is used:
|
|
The main changes to the dataset in terms of different expressions or methods used are summarized briefly here. Full details will appear in Holland & Powell (1998, J Met Geol, in press, May 1998).
1. Thermal expansion for solids.
The thermal expansion has been made a function of temperature such that, following Pawley et al. (1996, Am Min), a single parameter a° is used:
2. Compressibility of solids.
The Murnaghan equation of state is used with K'=4 and dK/dT = -0.00015 K used for compressibility (see Holland et al. 1996 Am Min). The EOS is given as
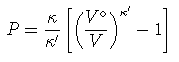
3. EOS for gases and fluids.
Use of the CORK equations for fluids (Holland & Powell, Cont Min Pet 1991) now extended to > 200 kbar using the data of Rice & Walsh (1957, J Chem Phys) for H2O and Sterner & Pitzer (1994, Cont Min Pet) for CO2
4. Order-disorder:
Phases which undergo order--disorder transitions or show lambda heat capacity anomalies were modelled using a tricritical Landau model in HP90. The approach has been extended here to include a pressure dependence, so that the order--disorder transition temperature can be a function of pressure. Adding a pressure dependence allows the behaviour of phases such as quartz to be modelled in a much more natural way, so that alpha and beta quartz do not require separate thermodynamic data. This is discussed more fully in Holland & Powell 1998 (J Met Geol, in press).
5. Hydrous cordierites.
New crd-H2O incorporates the new hydration data of Skippen & Gunter (1996, Cont Min Pet), and uses the Cp of Carey (1993, Phys Chem Mins) for hcrd and the enthalpy of hydration from Carey & Navrotsky (1992, Am Min). The level of agreement between the calorimetric data and the measured water contents makes a satisfactory justification of the assumption of ideal mixing of cordierite and hydrous cordierite with one mole of H2O, and lends weight to the model as a tool for calculating the thermodynamic properties of water-bearing cordierites.
6. Melt species.
The data for bulk modulus, entropy and enthalpy are derived from the PT experimental brackets on the relevant melting curves, constrained by entropy or enthalpy of fusion data, where available. For dry melts involving diopside, forsterite, fayalite, anorthite, albite, potassium feldspar, enstatite and quartz compositions, the melting curves are reproduced quite successfully to high pressures. For the H2O component in melts, the heat capacity and entropy are not known, and preliminary estimates are based on the properties of liquid H2O, adjusted on the basis of the wet melting of albite. The H2O end-member cannot be determined without recourse to mixing assumptions, and its properties are likely to need modification when the melt activity relations become better known.
7. Aqueous species.
The density model of Anderson et al. (1991, Geo Cos Acta) appears to be remarkably successful and we have extended the model to make it agree with not only the ambient-temperature solubility and calorimetric studies but also with the high-temperature and high-pressure mineral solubility experiments.
|
|